Date
2020-05-24Related Product
S-Patch Cardio
Arrhythmia Measurement Device Receives Certification in Europe and Begins Exporting
Healthcare company spun off from Samsung SDS
Obtained European certification last month... Exported to Iatly
Certification in Korea received last year
FDA approval application scheduled for July
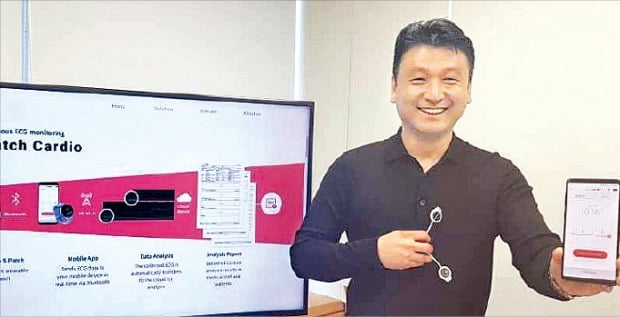
Young Juhn, CEO of Wellysis, introducing the wearable electrocardiogram (ECG) measuring device 'S-Patch Cardio'
Amid the revelation that most of the deaths from COVID-19 had underlying conditions, the mortality rate among individuals with cardiovascular diseases was found to be high. In light of this, Wellysis' wearable electrocardiogram (ECG) measurement device, the 'S-Patch Cardio,' which accurately and rapidly diagnoses cardiovascular diseases, received CE certification in Europe last month and was exported to Italy.
Longest 100-hour ECG measurement
According to Wellysis on the 24th, the S-Patch Cardio began its export to Europe starting with Italy in mid-April. It is also making preparations for export to Spain and Greece. Spain and Italy are the fifth and sixth countries with the highest number of confirmed COVID-19 cases, respectively, at over 230,000 and 220,000. Previously, Wellysis exported 30 units to New Zealand and 200 units to Thailand, confirming demand worldwide.
The S-Patch Cardio is a medical device that monitors ECG remotely by attaching it in patch form near the heart for up to 100 hours. Users can transmit ECG data measured in their daily lives to mobile devices such as smartphones and send them to a cloud-based web portal. This system supports medical professionals in diagnosing conditions such as arrhythmia based on the analyzed ECG data by artificial intelligence (AI).
Compared to the 'Holter' device, which has been primarily used for ECG measurement for the past 30 to 40 years and could conduct tests for 24 to 48 hours, the S-Patch Cardio increased the monitoring time to detect arrhythmias more effectively. By replacing the coin-type battery, readily available at convenience stores, the device can measure ECG for up to a month. According to the World Health Organization (WHO), the arrhythmia detection rate remains at around 30% for 24-hour tests but increases rapidly to over 70% after four days.
Unlike Holter devices that require multiple wires to be attached to the body and weigh 800g, the S-Patch Cradio weighs only 8g, making it lightweight and easy to attach, allowing users to conduct ECG tests during their daily activities. Unlike HUINNO's 'Memo Watch,' which was recently included in health insurance and measures ECG manually for a certain period after detecting heart abnormalities, the S-Patch Cradio can continuously measure ECG. Clinical trials have been completed in eight countries worldwide, including Korea, Australia, and the UK, and the results have been published in papers to verify its performance.
Targeting the global market including Europe and the United States
Wellysis is a company established in May last year by a digital healthcare team from Samsung SDS. Young Juhn, CEO of Wellysis, is a healthcare professional with over 20 years of experience in companies such as Johnson & Johnson.
During the Samsung SDS era, Wellysis developed the S-Patch Cardio by combining Samsung Electronics' bio-process chips with Samsung SDS's analysis and AI program technologies. It was verified through clinical trials at Samsung Medical Center. After completing product commercialization in 2018, Samsung SDS spun off the company to launch digital healthcare services.
7 months after founding the company, it obtained UHealth certification from the Korea Food and Drug Safety Administration, followed by certifications in Thailand in March and Europe and Australia in April. Certification work is also underway in Singapore and Vietnam.
Wellysis aims to expand into the untact (non-face-to-face) healthcare device market, estimated to be worth over 4 trillion won globally, beyond the wearable ECG measurement device market. CEO Juhn said, "The S-Patch Cardio is a product that transforms the concept of healthcare services from cure to care," explaining that "the goal is to diagnose and treat diseases using accumulated medical data." The company plans to divide the products into medical devices used in hospitals and those used in sports activities.
Juhn added, "We plan to apply for FDA certification in the United States in July after upgrading the product," and said, "We will expand exports to the United States, the largest market, and other countries around the world."
등록된 댓글이 없습니다.