Date
2023-03-22Related Product
S-Patch Ex
Wellysis will participate in the 38th International Medical Device & Hospital Equipment Exhibition (KIMES 2023), held at COEX in Seoul from the 23rd to the 26th of this month, to introduce its medical-grade wearable ECG solution, 'S-Patch Ex.'
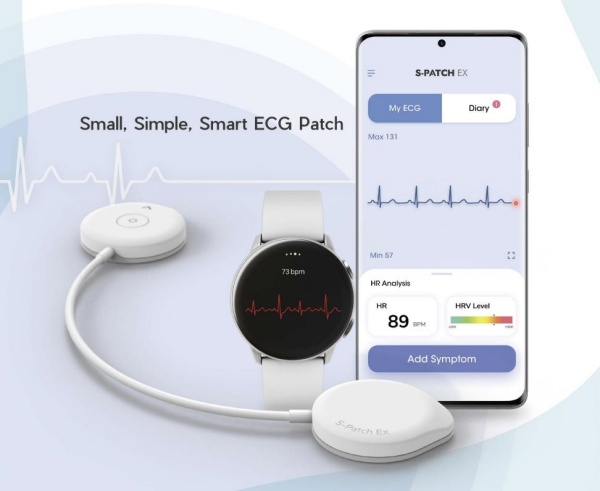
The S-Patch is designed as an ultra-lightweight patch weighing only 9g to minimize patient discomfort during long-term continuous ECG monitoring. It can be conveniently worn and used in daily life. With the ability to be continuously used for up to 3 days after patch attachment, it enables remote monitoring of ECG during use, significantly increasing the detection rate of arrhythmias for early diagnosis of cardiovascular diseases.
Moreover, real-time ECG and test progress can be monitored through the mobile app (S-Patch Ex). If any abnormal symptoms such as chest palpitations or chest discomfort are felt during the test, the symptoms can be recorded directly via the mobile app or the patch. After the test is completed, data can be transferred to a web portal through a cloud server, allowing healthcare professionals to access and interpret the data anytime, anywhere.
At KIMES 2023, Welysis will also unveil the healthcare professional-exclusive app 'S-Patch Medi' and the device 'S-Patch ExL,' which allows testing for over 72 hours. The S-Patch Medi app enables testing without the patient's smartphone, and it is compatible with various device lineups, including the existing S-Patch and the long-term testing-capable S-Patch ExL device, providing convenience in solutions.
Additionally, it is expected that conducting the entire process of ECG testing through one app will significantly enhance the efficiency of healthcare professionals' work. The S-Patch has obtained medical device certifications from domestic and international organizations, including the Korean Ministry of Food and Drug Safety, CE marking in Europe, and TGA in Australia. Moreover, it has been selected for the Johnson & Johnson innovation support program 'JLABS' this year, indicating preparations for entry into the U.S. market.
등록된 댓글이 없습니다.